Abstract
The goal of these two studies was to examine the measurements from two refractometers advertised to measure the total dissolved solids (TDS) content of coffee. Utilizing mixed study designs, the VST LAB Coffee II Coffee & Espresso Refractometer (the newer VST LAB Coffee-Espresso III will be tested soon) was compared against the Atago PAL-COFFEE refractometer in two different experiments. For the first study, distilled/demineralised water was used to zero the refractometer in one condition and brew water was used to zero in the other condition. Our results demonstrated no significant difference in the readings between the VST and Atago refractometers, nor between the two water types. Another experiment was performed to assess the impact of the syringe sample filters supplied by VST. Our results demonstrated a significant difference between refractometers (p = 0.04), a significant effect of filter use on TDS reading (p = 0.02), as well as a significant interaction between filter use and temperature (p = 0.01).
Disclosures
We have no vested interest in either product being tested.
Authors
Jeremy and Joe
Introduction
Coffee is most often thought of as a solution, consisting of a solvent (water) and solutes. As described in much greater detail by others (e.g., Flament 2002; Andueza et al. 2003), over 600 potential flavor-impacting compounds have been identified in the seed. While not all compounds contained within the roasted beans are soluble, the resulting complex beverage that is brewed is considered by many a homogenous mixture composed of a single phase with particles that cannot be seen by the naked eye, thus making its soluble content quantifiable through standard means of solution concentration measurement such as refractive index.
In simplistic terms, the index of refraction describes how light passes through a medium. The angle of refraction as light passes through the coffee (i.e., how the light bends when passed through the solution) is often captured by a photodetector and the resultant refractive index calculated. This is a method typically used to assess the concentration of a solute in an aqueous solution, most often called the total dissolved solids (TDS) percentage (for more in-depth explanation on refractive index and its application to coffee, see Clarke 1989).
At least two consumer-grade refractometer product lines have been marketed specifically for coffee solute concentration assessment: a series marketed by VST and a series produced by Atago. Both have offered several models. Early iterations of what would become the VST refractometer were produced by Atago, who has been developing refractometer technology for over 70 years. VST then switched to Reichert Techologies refractometer hardware (Reichert has a 150-year history of producing optical measurement equipment), originally called the R2 mini but now sold as the Brix/RI Chek. The current model tested here is based on the hardware of the Misco Palm Abbe. VST branded equipment has custom-loaded software on its refractometer units to provide a TDS of coffee/espresso directly without the need for correlation tables. Atago has been marketing their own coffee-specific refractometer calibrated with an “industry scale” specifically for coffee, providing a direct readout of either Brix (a scale based simply on the refractive index of sucrose in an aqueous solution) or correlated TDS reading. For anything other than sucrose, the Brix scale only provides an approximate dissolved solid content reading. VST recommends the use of a syringe filter for certain non-paper filtered coffee brews or espresso. Atago does not make any suggestions regarding the use of a sample filter for filter brewed coffee or espresso.
We set out to test the performance of the two devices compared against each other, not assess their individual precision or accuracy against stated manufacturer specifications. To test precision and accuracy within a device would require us to compare against another metric of TDS quantification and a known reference solution.
Methods
The VST device used was the LAB Coffee II (Coffee & Espresso) Refractometer (stated range: 0.00-20.00%; TDS resolution 0.01%; precision +/- 0.03%; accuracy from 0.00-4.99% of +- 0.05% and from 5.00-20.00% of 0.10%; temperature range of 15-40C) and the Atago device was the PAL-COFFEE (stated range: 0.00-25.00% Brix; TDS resolution 0.01%; temperature range of 10-100C). Both were loaded with fresh batteries and warmed to room temperature (25C).
Distilled water, using a conductivity-based TDS meter, contained 0 ppm. The brew water used contained 94 ppm. Both of these registered as 0.00 on the VST and Atago.

The coffee used for the experiment was Toby’s Estate fully washed Rwanda Ruli roasted for filter coffee.
Equipment used:
- Kalita stainless steel wave dripper
- Kalita filter paper
- Kalita carafe
- Bonavita 1.0 L digital variable gooseneck kettle
- Infrared gun
- FLIR thermal camera
- AWS scale
- HGone Conical Manual grinder
- Atago refractometer
- VST refractometer
- 2 new sets of batteries
- Pipettes
- Beakers
- Alcohol wipes
- Ceramic cups
- Tissues
Study 1: Which Water to Zero Your Device
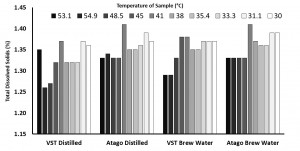
Pour over coffee was brewed at 94 degrees Celsius at a ratio of 1g coffee to 17g water. The brew was 15g of coffee to 220ml of water, with a total time of 2 minutes and 45 seconds. No stirring or agitation was applied during the brew. The coffee was set to cool for approximately 1 minute after brewing before the start of the experiment. Two separate beakers and pipettes were set up in place, one containing distilled water and the other tap water (referred to throughout as “brew water”) to zero both refractometers at the start of each round prior to the TDS recording. Each round consisted of 4 TDS readings in this order: VST coffee sample TDS measurement with the device being zeroed on distilled water; Atago coffee sample TDS measurement with the device being zeroed on distilled water; VST coffee sample TDS measurement with the device being zeroed on brew water; Atago coffee sample TDS measurement with the device being zeroed on brew water. Prior to every zeroing and TDS recording for each round (10 total rounds, 40 total readings), the lens for each device was cleaned with alcohol wipes. As a sample of coffee was drawn from the carafe, the brew temperature was taken (and verified with multiple devices). The temperature of the sample was taken again when it was transferred to a ceramic cup before being dispensed on the refractometers.
Testing procedure:
Prior to each round, the brew was stirred in the carafe and the temperature was taken and recorded. For each ceramic cup, enough coffee was drawn for all sampling in the round (4 total). Further, the pipette was rinsed with the coffee solution, as per VST recommendations. Two new pipettes were allocated for each round to siphon the coffee inside the ceramic cup. All samples were rested on the refractometer lens for approximately 20 seconds before measuring the TDS. It is important to note that the Atago refractometer took longer to display the readings.
Study 2: Impact of the VST Syringe Sample Filters
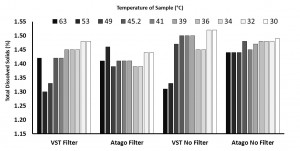
Another pour over coffee was brewed at 94 degrees Celsius at a ratio of 1g coffee to 17g water. The brew was 15g of coffee to 220ml of water, with a total time of 2 minutes and 45 seconds. No stirring or agitation was applied during the brew. The coffee was set to cool for approximately 1 minute after brewing before the start of the experiment. Two groups of sample syringes were set up: one group fitted with the sampling filters; one group not fitted with the filters. Devices were zeroed using the brew water. Each round consisted of 4 TDS readings in this order: VST coffee sample TDS measurement with the sample being passed through a VST sample filter; Atago coffee sample TDS measurement with the sample being passed through a VST sample filter; VST coffee sample TDS measurement with no filter on the sample; Atago coffee sample TDS measurement with no filter on the sample. Prior to every zeroing and TDS recording for each round (10 total rounds, 40 total readings), the lens for each device was cleaned with alcohol wipes. As a sample of coffee was drawn from the carafe, the brew temperature was taken (and verified with multiple devices). The temperature of the sample was taken again when it was transferred to a ceramic cup before being dispensed on the refractometers.
It is worth noting VST states the filters should be used for “clarifying espresso prior to refractometer measurements. Also suitable for use with COFFEE ground for filter brewing using perforated metal or woven mesh filters.” As such, our test beverage brewed with a paper filter should not need the filters, according to the VST guidelines.
Testing procedure:
Prior to each round, the brew was stirred in the carafe and the temperature was taken and recorded. For each ceramic cup, enough coffee was drawn for all sampling in the round (4 total). Further, the syringe was rinsed with the coffee solution, as per VST recommendations. Two new pipettes were allocated for each round to siphon the coffee inside the ceramic cup. All samples were rested on the refractometer lens for approximately 20 seconds before measuring the TDS. It is important to note that the Atago refractometer took longer to display the readings.
Results
Study 1: Which Water to Zero Your Device
In the “instrument zero based on distilled water” condition, the VST average TDS was 1.33 with a standard deviation of 0.06 and the Atago average TDS was 1.36 with a standard deviation of 0.03. In the “instrument zero based on brew water” condition, the VST average TDS was 1.35 with a standard deviation of 0.03 and the Atago average TDS was 1.36 with a standard deviation of 0.03. A generalized linear model was run with the independent variables (2×2 between variables: VST/Atago; Distilled/Brew Water; co-variate: Time/Temperature for each sample). There were no significant findings at the p < 0.05 level (Refractometer: F(1,8) = 4.24, p = 0.07; Water Type: F(1,8) = 0.13, p = 0.73).
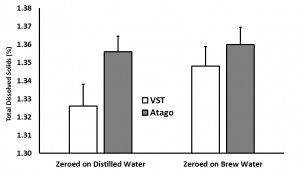
Study 2: Impact of the VST Syringe Sample Filters
These samples were taken from a new batch of coffee, brewed solely with the same tap water used in the previous study.
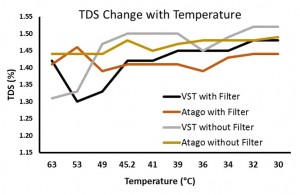
In the “samples with syringe filter” condition, the VST average TDS was 1.42 with a standard deviation of 0.06 and the Atago average TDS was 1.42 with a standard deviation of 0.02. In the “samples with no syringe filter” condition, the VST average TDS was 1.46 with a standard deviation of 0.08 and the Atago average TDS was 1.47 with a standard deviation of 0.02. A generalized linear model was run with the independent variables (2×2 between variables: VST/Atago; Filter/No Filter; co-variate: Time/Temperature for each sample). A significant difference between refractometers was found, F(1,8) = 6.32, p = 0.04, as well as a significant difference in TDS with the use of a filter, F(1,8) = 10.3, p = 0.01, and a significant interaction between the use of a filter and the temperature of the sample, F(1,8) = 11.2, p = 0.01). Examining the interaction more closely, as temperature cooled, the impact of using a filter decreased (this appears to be driven mostly through variability in the VST readings at warmer temperatures).
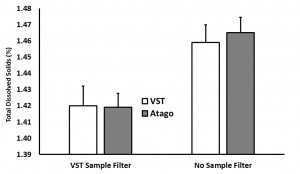
(Raw data can be downloaded in a tab delimited text file here. As always, while we offer the data for your personal use, we kindly ask that you send a message to socraticcoffee@gmail.com before posting or presenting it in any public forum and attach appropriate acknowledgement.)
Conclusions
Overall, it appears the VST and Atago refractometers are very comparable for measuring the TDS of filter brewed coffee. While the second study examining the impact of the sample filter demonstrated a large amount of variability in the VST readings compared to the Atago, this was not seen in the first study (distilled/brew water; VST standard deviation 0.04; Atago 0.03).
Within a device, there was not a significant difference using distilled water to zero versus using brew water. However, it is worth noting that the brew water for our experiment was much lower than others may use (94 ppm). The purpose for which a person uses a refractometer with his/her coffee may influence the best approach to zeroing their device. It is possible that brew water could be of high enough mineral content to register on the VST or Atago refractometer and that brew water TDS levels may vary from time-to-time and/or place-to-place. Because this is the case, zeroing based on brew water would allow for a useful, relative metric with TDS (e.g., 1.35% zeroed on brew water would mean 1.35% to anyone else who also zeroed on brew water). It is solely a measure of the soluble content contributed by the coffee. This differs from zeroing based on distilled water, which does not allow for a differentiation between soluble content of initial water and soluble contribution from the coffee itself. Further, as zeroing on brew water does not alter the device hardware but instead changes the software set point for the case-at-hand (and can be returned to “absolute zero” with distilled water at any point), the brew water zeroing approach has a practical justification. The argument that a non-distilled water zero will decrease accuracy of the device is not supported in our data and we welcome open and honest data that shows otherwise. While perhaps only extreme brew water mineral content start points might register on these devices and/or, in psychophysics terms, breach the “just noticeable difference” in human perception, until objective empirical data demonstrates a substantiated issue caused by using anything-other-than-distilled-water to zero your device, we argue a good practice would be to zero the device based on the true starting condition (i.e., brew water).
Using the VST sample filters significantly decreased the TDS for both refractometers, but their use created greater variability in the VST readings. Our data cannot suggest whether a person should use or not use the filters. While their purpose is for beverage preparations other than the one used in this experiment, they do appear to have a significant effect on overall TDS (decrease of soluble content). How that improves the accuracy of the TDS reading is not clear. Again, from a practical perspective, the decision to filter out particulates of the solution being measured will most likely depend on how a person intends to use the refractometer. If the intent is to assess the soluble concentration of a brew as-is, no filter seems logical. If it is to assess the soluble concentration of a brew, cutting off particles of a certain size, the filter would be appropriate. This experiment did not assess readings from espresso, which may be more heavily impacted by the use of a filter. Many methods of coffee preparation, no doubt, include varying degrees of sediment and colloidal material. Whether or not it is “accurate” to remove particles before assessing the TDS of coffee/espresso seems, at this point, to be more of a subjective decision.
Overall, it is important to understand what refractometry does and does not tell you about the coffee. Because the light is manipulated as it passes through a substance at a rate proportional to the substance’s concentration in the solution, refractometry in coffee, which is a mixture of many compounds, can only give a metric for the cumulative effect of the various constituents. Essentially, the refractometer gives you a glimpse into how much stuff is in there, but nothing about what that stuff is composed of. Further, temperature is clearly an important factor for refractometry (i.e., temperature changes the density of water, which changes its refractive index). It is also an important factor for the refractometry of coffee, specifically, since some compounds may be soluble at higher temperatures but precipitate out of the solution as it cools. For now, the use of a refractometer seems best suited as a tool for quantitatively tracking coffee preparation consistency and, whichever methods are chosen (e.g., distilled zeroing, use of sample filter), it is important to maintain them for comparability.
References
Flament I. (2002). Coffee Flavor Chemistry. West Sussex, England: John Wiley & Sons, Ltd.
Andueza S., Maeztu L., Pascual L., Ibanez C., Paz de Pena M., Cid, C. (2003). Influence of extraction temperature on the final quality of espresso coffee. Journal of the Science of Food and Agriculture, 83, 240-248.
Clarke R.J. (1989). Water and Mineral Contents. In R.J. Clarke & R. Macrae (Eds.), Coffee: Chemistry (Volume 1) (pp. 42-82). New York, NY: Elsevier Science Publishers LTD.







coffeecircus
Thank you very much in carrying out this experiment!
Joe
It is great pleasure and thank you for taking the time to read the review!
Tom Chips
Thank you for all the work that went into this! Do you plan on doing a similar comparison with espresso strength extractions?
Joe
Many thanks for the feedback…hopefully in due course we will do it. In having said that We need to be able to control few variables, like tamping, brewing temperature etc…Challenging but feasible!
Steve
Fantastic article. Thanks for the time dedicated to this!
For Atago, it seems that temp has little impact on TDS. Correct? Or would there be a temp best suited for TDS reading?
Joe
According to the above data & graph, the TDS (Atago without filter) reacted differently to the temperature, it increased at 45.2 Degrees and then slightly decreased and thereafter maintained consistency in the reading. It appears that lower temepratures yielded more consistency in the TDS reading for the Atago without filter.
cembozkus
jeremy
enable permanent links from wordpress settings. so google can index your posts.
great job. it will be massive.