| Main Questions
1) How does particulate matter affect refractive index (RI) measurements? 2) Do VST filters affect sample content (nondissolved solids or otherwise)? Abstract Nondissolvable, light-scattering solids (i.e., titanium dioxide, TiO2) were added, in increasing amounts, to various solutions. The refractive index-converted-to-coffee TDS of samples from each solution, using an Atago PAL-COFFEE and VST LAB Coffee III, were measured with and without passing through a VST syringe filter. Results suggest a significant effect on TDS measurement as the amount of TiO2 increased (p=0.00). Though the TDS differed between solutions (p=0.00), the interesting finding was that the impact of the TiO2 differed based on the solution being tested (interaction effect, p=0.01). Lastly, a significant effect of the filters was observed (p=0.00). Bottom line: The VST filters do appear to remove much of the particulate matter (though, not all and not consistently across the various solutions). Both refractometers were affected by the addition of particulate matter and their readings did not differ significantly from one another. Lastly, while the VST filters appeared to reduce TDS content in the brewed coffee and instant coffee solutions, we also found they appear to release some particulates which register as dissolved solid content (a finding we confirmed doing a simple elution study discussed in the Conclusions section). Disclosures We have no vested interest in any of the products used for this experiment. Authors Jeremy, Joe, & Dave |
Introduction
Refractometers work on the principle of refractive index (RI). For a clear, coffee-related explanation, we suggest reading this explanation. To think of it simply, a refractometer consists of a light emitter and a light detector. The sample is placed between these two, allowing for some deviation in the expected path of the light (i.e., your zeroed condition). Since the bend of the light through the solution is absolutely critical for measurement accuracy, particles that interfere with the passage of the light are undesirable. Enter titanium dioxide (TiO2). Used in a variety of applications to include paint, sunscreen, and food coloring, among its numerous interesting properties, TiO2 is well-known for its high efficiency to scatter light via its high refractive index and small particle size. Think of the refractive index in a sample as your “signal” and TiO2 as deliberate “noise”.
Particulate matter—or anything that does not transmit light (e.g., nondissolved solids)—should not, theoretically, contribute to a solution’s RI since it does not bend the light. Unfortunately, this is not so clear cut as these particulates may prevent light from cleanly transmitting from the emitter to the detector. Further, the assumption that the particulate matter is not transmitting any light may not be entirely correct. Though our previous work with the VST filters suggested that the total dissolved solids (TDS) content of espresso actually decreased when the filters were used–we considered that this could be due to light scattering affecting the detector/algorithm. And, perhaps, our measurements were suffering from noise caused by that scattering. Particulate matter whose size approaches the wavelength of light in the refractometer’s emitter (e.g., yellow light, as in what Atago/VST most likely use, corresponding to 570-590 nm) will increasingly scatter light. Such scattering, although it does not directly concern RI, can perturb its detection. Much of this depends on the detector, amount of particulate, and how the actual detector optical qualities and software respond to such sample noise.
Pigment grade titanium dioxide also presents a very high RI (about 2.6), so, as mentioned previously, it does refract some light. Things get complicated when you have a large RI mismatch between suspended particles, like TiO2 and water (RI = 1.3). The first solution tested (distilled water only) is to observe how distilled water plus TiO2 affects measured RI. This is a control condition, adding only insoluble material (i.e., TiO2). Subsequent solutions incorporated additional soluble or soluble/insoluble compounds. The TiO2 used here has a stated minimum size of 100 nm (.1 µm). We were unable to get a maximum particle size declaration from the manufacturer. Dissolved solids are often defined as particles which pass through a 2 µm filter, which is the assumed pore size of the VST syringe filters.
Hypotheses
- The addition of TiO2 will increase light scattering, leading to increasingly altered readings from baseline with the refractometers.
- Removal of particulate matter with the VST syringe filters will increase signal-to-noise ratio within the sample, allowing for a more consistent reading (i.e., similar to the “no TiO2” condition).
Methods
Equipment:
- TiO2, pigment grade, 500 g (New Directions Australia)
- “WT” Demineralized water
- EasyLog TH+ room temperature logger
- 4 X 2L Plastic containers with Lids
- 32 x 100 ml ramekins (for storing solutions)
- 32 x 40 ml ramekins (for storing samples)
- 60 ml syringes
- 10 ml syringes
- Spoons
- VST syringe filters
- Atago PAL-COFFEE refractometer
- Atago RX007α
- VST LAB Coffee III refractometer
- Alcohol wipes
- Ohaus Navigator scales
- Ohaus Pas 213 (0.001 g to measure the TiO2/instant coffee/sugar/coffee)
- Plastic lab stirrers
- Nescafe Classic Instant coffee
- White table sugar
- Chemex & filter paper
- Bonavita kettle
- Pen and paper to record values
Procedures
Four solutions were created, all starting with distilled/demineralized water:
Condition 1 contained only distilled water
Condition 2 contained distilled water + sucrose (white table sugar)
Condition 3 contained distilled water + instant coffee (100% dissolvable solids)
Condition 4 contained brewed coffee made with distilled water
All kettles were rinsed with demineralized water prior to boil. For all conditions, the distilled water was first boiled. Condition 1 served as the control, so other than the light-scattering agent (described below), nothing was added. Condition 2 was made with 10 g of white table sugar to 400 g of just-off-boil distilled water (yielding a calculated Brix of 2.43% and measured TDS of 1.97% [average between Atago and VST]). Condition 3 was made with 10 g of instant coffee to 400 g of just-off-boil distilled water (yielding a measured TDS of 2.215% [average between Atago and VST]). Condition 4 was made of 57.17 g of coffee brewed to yield a final beverage mass of 400 g (yielding a coffee TDS 1.585% [average between Atago and VST]). Each solution was covered and left to cool overnight (temperature was recorded for the night—maintaining just under 25°C).
Solutions were then divided into eight (8) 50 g containers, with each container labeled 1 through 8. Container #1 as used as the baseline, with nothing added. In container #2, 0.1 g of TiO2 (yielding a concentration of 0.2%) was added. In container #3, 0.25 g of TiO2 (0.5%) was added. In container #4, 0.5 g of TiO2 (1.0%) was added. In container #5, 1 g of TiO2 (2.0%) was added. In container #6, 2 g of TiO2 (3.8%) was added. In container #7, 4 g of TiO2 (7.4%) was added. In container #8, 8 g of TiO2 (13.8%) was added. This created 32 total containers (8 per solution), with 28 of them having varying amounts of TiO2.
Before a 2 ml sample was drawn from a container, the solution was always stirred. Samples were drawn with and without the use of a VST syringe filter and place into dedicated sample ramekins. Refractometers were cleaned thoroughly, first with demineralized water, and then with alcohol pads between each reading.
Results
Data was stripped of identifiers related to solution, refractometer, and condition order (filter/no filter), and matched with a key after results. The data set was then checked for violations of assumptions for generalized linear model/ANOVA. A mixed model ANOVA was then run using the between variables of refractometer (Atago/VST) and solution (distilled, sucrose, instant, brewed), as well as within variables of condition (filter/no filter), sample (TiO2 concentration). Significant main effects were observed with solution (F3,119=91.05, p=0.00), condition (F3,119=75.83 p=0.00), and sample (F1,119=17.32, p=0.00). No significant differences were found between refractometers. A significant interaction was observed between sample and solution (p=0.01). Data was collapsed across refractometer for further analyses.
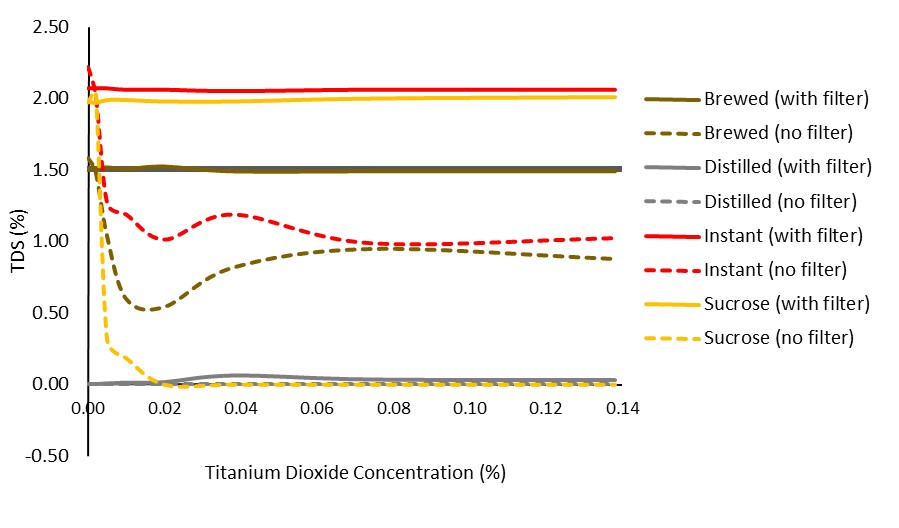
Figure 1. Impact of VST syringe filters in various solutions across varying amounts of TiO2.
It is clear that the filters allowed for a more consistent reading (improved precision). It is not clear, however, if they aided in a more accurate reading. To look at the data another way, we averaged the TDS reading for each solution, highlighting the filters’ ability to block added noise (i.e., reduce standard deviation):
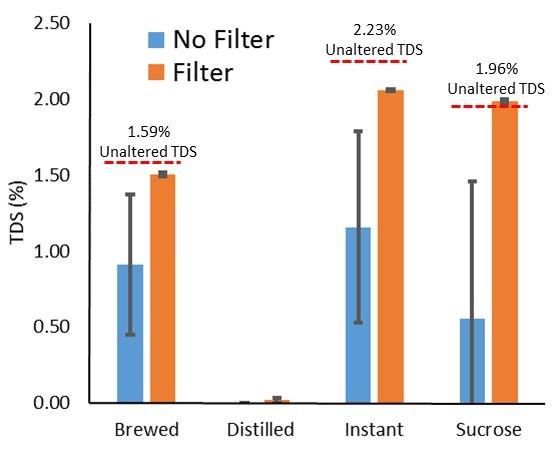
Figure 2. Collapsed across TiO2, demonstrating syringe filters reducing reading variability.
The above figure shows a significant effect of filtering on reducing noise in the sample/variability in the reading. One note is the slight reduction in readings seen only when coffee solubles were present in a solution (instant or brewed). This is evident at the 0.0% TiO2 level, but remains as TiO2 level increases.
Further, we did notice readings appeared in the distilled only solution after filtering (a slight increase is also seen in the filtered sucrose solution). Because of this, we did a simple elution (rinsing) experiment described in the Conclusions section.
(Raw data can be downloaded in a tab delimited text file here. As always, while we offer the data for your personal use, we kindly ask that you send a message to info@socraticcoffee.com before posting or presenting it in any public forum and attach appropriate acknowledgement.)
Conclusions
The main finding of this study is that the VST filters produced consistent results from solutions, even with increasing noise. It is worth noting that the levels of noise created by the TiO2 most likely far exceeded that found normally in coffee/espresso. Further, coffee, whether instant or brewed, regularly demonstrated a decrease in TDS after passing through a filter. This is evident even at the 0.0% TiO2 level, suggesting, as our previous work has, that the VST syringe filters remove some amount of dissolved solid content. It is also worth noting that the effect of the TiO2 on TDS with filtering was not consistent for all solutions (that is the significant interaction effect observed of Solution x Sample). Some, but not total removal, may suggest particle size variability in the TiO2 used, potentially exceeding the pore size of the filters. Further, to explain the differing effects of TiO2 on the various solutions, we propose two processes that may be occurring:
- Change in the refractive index of TiO2: Coffee solubles may be getting absorbed to the TiO2 particles, coating them and thereby reducing transmission of light through the particles. This would imply the particles become more opaque and the TiO2’s high RI is no longer able to effectively scatter light.
- Scatter/diffraction: As more TiO2 is added, perhaps the coffee components coating the TiO2 particles are titrated out. Coated particles may be aggregating and presenting a high level of light attenuation as well as scatter.
Small Elution Study
To more closely examine the possibility that the VST filters release something into a sample which may register as a dissolved solid, we pushed equal amounts of 90°C distilled water through new VST syringe filters twice (2 ml each time), capturing each “rinse” of the filter in its own container (the “eluted” sample). The liquid was allowed to cool to room temperature before measurement. Besides the two previously mentioned refractometers, we also included the Atago RX007α. This table summarizes our observations (5 samples per average). It appears the VST filters release some particulates into the sample which register as dissolved solids (i.e., positive TDS readings with distilled water only).
| Distilled Water | |||
| No Filter | Rinse #1 | Rinse #2 | |
| Refractometer | Avg (SD) | Avg (SD) | Avg (SD) |
| Atago PAL-COFFEE | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) |
| VST LAB Coffee III | 0.0 (0.0) | 0.014 (0.01) | 0.01 (0.00) |
| Atago RX007α | 0.0 (0.0) | 0.00272 (0.002) | 0.00484 (0.000) |
The above readings should all be 0.00. It is evident the VST syringe filters release something into the sample which registers as a dissolved solid by at least two of the refractometers. The lower reading of the Atago RX007α may be due to a limitation in our refractive index-to-coffee TDS conversion.
We then repeated this experiment but used two strengths of instant coffee (one near espresso strength, 8.4% TDS, and one near filter brewed coffee strength, 1.28% TDS). Instant coffee claims to be 100% dissolvable solids, which would mean it, theoretically, would not be affected by 2 µm filtering/VST syringe filters. Non-filtered readings were taken, then filtered readings, and then readings from the resultant solution when the used filters were eluted. This table summarizes our observations (5 samples per average).
| Instant Espresso Strength | |||
| No Filter | Filtered Sample | Eluted Sample | |
| Refractometer | Avg (SD) | Avg (SD) | Avg (SD) |
| Atago PAL-COFFEE | 8.4 (0.01) | 8.35 (0.07) | 7.09 (0.58) |
| VST LAB Coffee III | 8.4 (0.01) | 8.28 (0.06) | 7.12 (0.58) |
| Atago RX007α | Out of Range | Out of Range | Out of Range |
With the high sensitivity of the Atago RX007α, values in the “espresso range” exceeded its specifications for upper refractive index limit.
| Instant Filter Strength | |||
| No Filter | Filtered Sample | Eluted Sample | |
| Refractometer | Avg (SD) | Avg (SD) | Avg (SD) |
| Atago PAL-COFFEE | 1.32 (0.01) | 1.31 (0.01) | 0.56 (0.07) |
| VST LAB Coffee III | 1.25 (0.02) | 1.31 (0.01) | 0.56 (0.05) |
| Atago RX007α | 1.53525 | 1.32142 (0.0052) | 0.58181 (0.0563) |
Again, the differing coffee TDS values between the Atago RX007α and VST LAB Coffee III may be due more to our conversion. We cannot use this data to determine the accuracy of one device over the other (even though the Atago RX007α is stated by the manufacturer to be much more sensitive to changes in refractive index than the VST LAB Coffee III). The more important point here is that the filters, in the brewed coffee strength instant coffee, reduce coffee soluble content as a sample passes through in two of the three refractometers tested. These solubles can then be released from the filters via elution, which can also be quantified. To more clearly demonstrate this finding, dehydration of a filter after a sample has passed through should be performed. It is also unknown how much of the released solubles are from the filter itself and how much came from the solution. With the VST LAB Coffee III readings increasing post-filter, we believe it may point to a correction factor in the device’s detection algorithm. Further, because lipids would not be present in instant coffee, the deviation may also reflect some algorithm-based correction for their expected presence in the VST refractometer.












Matt Hoffman
This is a layman’s question. I have an Atago PAL Coffee refractometer. Does your work indicate that I should filter expresso samples? At the moment, I don’t.
Thanks very much.
Jeremy
Hi Matt,
Unfortunately, the decision to filter or not filter your coffee (I’m using that term to mean espresso as well), is one that we cannot say you should or shouldn’t do. The important thing is to only compare readings collected the same way. Our work suggests the VST syringe filters do significantly lower TDS. Looking at it more closely, we see that the filters retain some dissolved coffee solids as well as release something (not coffee) that can register as a dissolved solid (unluckily, this is not a 1-for-1, as the filters retain more than they release). We have asked Atago why they do not recommend filtering coffee and here is their response, “By using those filters and syringes the espresso is no longer the same as what people actually drink. Then we thought, what does the Brix (TDS) value after filtration mean?” This is a valid point and something to consider. Atago and MISCO (the VST device manufacturer), and lots of other manufacturers, make refractometers with a variety of industry scales. Interestingly, we have not found any of them selling sample filters. VST suggests the syringe filters’ use is to remove noise in the sample and create a more stable reading. But one might ask, “A more stable reading of what?”
Personally, I do not filter my samples.
Hope that helps,
Jeremy
JK
Hi Jeremy, do you still maintain the stance of not filtering your (I preume espresso) samples?
Jeremy
Hi Jason — thanks for the question. For the most part, yes. The truly important part is that whatever you choose to do, you continue to do it for all samples so that you are always comparing apples-to-apples. It’s largely a choice between external and internal validity. As a refractometer reading should *theoretically* only be disturbed by dissolved solids, the argument could be made that filtering is unnecessary. Further, a non-filtered sample more realistically represents the actual beverage being consumed. However, from a scientific measurement/purity/internal validity perspective, filtering clearly affects the sample (as we have demonstrated many times) and could may be providing a more “clean” reading of dissolved solids. Changes seen in distilled water readings after using filters is a bit concerning, making the latter point a bit questionable–they don’t appear to be benign when it comes to affecting supposed dissolved solid content in a sample.
Jeremy
Pedro Ros Casanova
Good job, bad instant coffee. 🙂
Jim
Is it possible to take the RI measurement from Atago RX007α and use VST CoffeeTools to get TDS and an extraction yield? As far as I understand VST put that part of the computation to be performed on the device but I suppose there is no reason it couldn’t be done in the app itself?
Jeremy
Hi Jim,
I’m not familiar with a spot in the VST CoffeeTools where we can directly enter a refractive index measurement. I know that you can edit dose, beverage mass, % TDS, LRR, CO2, and moisture. The refractive index (nd) is converted to a coffee dissolved solids (% TDS) measurement via the proprietary software correlation tables running on top of the MISCO hardware. But maybe there’s something we’re missing….?
Jeremy
Will
When I was first learning how to use a refractometer from folks at BUNN, I was taught to pass brewed coffee through another sheet of paper filter (pre-rinsed with the same brewed coffee), as it is being dripped onto the lens of the refractometer. What are your thoughts on this approach?
Jeremy
Hi Will,
Seems like a bit of extra (possibly unnecessary?) work. There is a common “operational definition” of what constitutes a dissolved solid, suggesting it’s anything small enough to fit through a 2 micrometer pore filter. Passing your sample through a paper filter, one that has been pre-rinsed with the coffee being tested, is probably intended to wash away non-brew related materials via the pre-rinse and then act as a 2 micron filter so that only “dissolved solids” remain. Odds are that the paper filter does not have uniform 2 micron pores, so its efficacy in this application is questionable. However, as a good practice procedure, the pre-rinse idea for any intended filter may be a good one, especially if using the VST syringe filters, as they were shown to release materials that affect refractive index. Though, the whole idea of needing to filter is questionable since refractometers need a substantial amount of “non-dissolved solid” noise to alter the reading–and this doesn’t touch on the practical rationale against filtering in general. It’s also worth pointing out that, similar to taring a scale, your refractometer is intended to measure “change from starting brew water”, so it should be zeroed on your brew water before measurement, not distilled water like most people choose to do. Lastly, different fields may define “dissolved solid” differently, so the “2 micron” cut-off may not be a good one for coffee. Refractometers have been around a LONG time, so I’d recommend reading how other disciplines approach sample preparation. Whatever your sampling technique, the bigger thing is to make sure you are consistent for all samples being measured.
Best,
Jeremy
Gints
Do you know what is the pore size of VST syringe filters? 0,22um or 0,45um or some other?
Jeremy
Unfortunately, we don’t have that information. If you’re able to track it down, please let us know.
Meris Prosic
It’s 0,45μm in case you still don’t know
Jeremy
Thanks! That’s great info. Is that documented somewhere that you can share with us?
Chris Russ
Hi Meris,
Thermofisher make syringe filters at both 0.2and 0.45 micron for a better price than VST. I
‘ve wanted to confirm the sizing too. Do you have have any documentation as Jeremy asked? I’d love to confirm it before trying.
http://www.thermofisher.com.au/Uploads/file/Scientific/Laboratory-Plasticware-Glassware-Supplies/Filtration-Products/1520291070_Sartorius_Filtration_Final_AU_Brochure.pdf
James Reid
Interesting research.
As a serious coffee amateur roaster and barista, I use optical refractometers to dial in grind and brew ratio. Generally I start at 1:17 for immersion or vacuum-brewed coffee, take a reading (brix), multiply that by 0.85 (Andy Schecter, I think, came up with this conversion, and then use the SCAA chart to determine tds.
Not a Q-taster to be sure, my experience is that this does enable me to brew very good coffee. A change in dry weight of as little as 0.2g affects readings and flavor. Roasting also affects optimum ratio, it seems.
I’ve less experience using the optical device with espresso. That’s because I became aware of the filtering idea and figured my readings with espresso were likely unreliable with the greater amount of solids present. I considered using a centrifuge as those VST filters are too expensive for non-professionals as myself to use regularly (as are the Atago and VST digital devices).
What do you think? Am I nuts to be using optical devices? I do wonder how their readings would compare with the digitals. I also take note of your advice re: to filter or not– that the main point is consistent methodology.
I’d be interested in any thoughts you might have on the subject.
Jeremy
Hi James,
I believe (but could be wrong) the 0.85 Brix multiplication was actually from Alan Adler (of Aerobie/Aeropress fame) since he was the first to start using a refractometer to assess the strength of coffee in recent memory. Alan’s early work is what helped get Vince started with his refractometer coffee correlation tables. Some of those old posts in the Home Barista and CoffeeGeek forums have been taken down since Vince received his patent.
Regardless, your approach is sound if you’re simply using it as a relative comparison tool. So if you always use the same measuring technique and compare your numbers to your other measurements, that should be fine. It’s when you try to compare with readings from a digital refractometer with built-in coffee correlation tables that you’ll possibly run into some issues. Also, the optical device will lack the resolution that many feel they need in coffee TDS assessment, but if you’re happy with what you can achieve using the optical device, then you should be good to go. Do you need, for example, the ability to discriminate 1.35% TDS to 1.37% TDS? Hard to say. Can a person reliably taste that difference? I would be very skeptical. Lastly, though not great, many of the digital refractometers include a temperature correction for the readings since temperature can greatly impact the result. On one of our Instagram posts, we posted a Brix to coffee TDS conversion formula… it is polynomial and really needs to be repeated at many different temperatures (since the correlation curve changes at different temps), so it might just be easier to stick with your 0.85x conversion and know that you’re “in the ballpark”. Really it comes down to: what are your goals with TDS assessment and what level of measurement precision do you need to achieve those goals?
Best,
Jeremy
James Reid
Thank you, Jeremy. For me the ultimate parameter is the taste of the coffee. My main limitation is limited organoleptic experience, but I actively work on that, tasting various coffees prepared by reputable roasters and baristi as often as circumstances permit. I’ve certainly had some memorable experiences this way, and strive to re-create experiences as compelling with my roasting and brewing. Not always successful, yet I feel gentle growth occurring and am drinking and sharing better and better– if not perfect– coffees.
I appreciate your taking time to reply. Thanks!